- Thyroid
- Different Molecular Phenotypes of Progression in BRAF- and RAS-Like Papillary Thyroid Carcinoma
-
Jinsun Lim, Han Sai Lee, Jiyun Park, Kyung-Soo Kim, Soo-Kyung Kim, Yong-Wook Cho, Young Shin Song
-
Endocrinol Metab. 2023;38(4):445-454. Published online July 18, 2023
-
DOI: https://doi.org/10.3803/EnM.2023.1702
-
-
1,818
View
-
103
Download
-
1
Crossref
-
 Abstract Abstract
 PDF PDF Supplementary Material Supplementary Material PubReader PubReader  ePub ePub
- Background
Papillary thyroid carcinoma (PTC) can be classified into two distinct molecular subtypes, BRAF-like (BL) and RASlike (RL). However, the molecular characteristics of each subtype according to clinicopathological factors have not yet been determined. We aimed to investigate the gene signatures and tumor microenvironment according to clinicopathological factors, and to identify the mechanism of progression in BL-PTCs and RL-PTCs.
Methods
We analyzed RNA sequencing data and corresponding clinicopathological information of 503 patients with PTC from The Cancer Genome Atlas database. We performed differentially expressed gene (DEG), Gene Ontology, and molecular pathway enrichment analyses according to clinicopathological factors in each molecular subtype. EcoTyper and CIBERSORTx were used to deconvolve the tumor cell types and their surrounding microenvironment.
Results
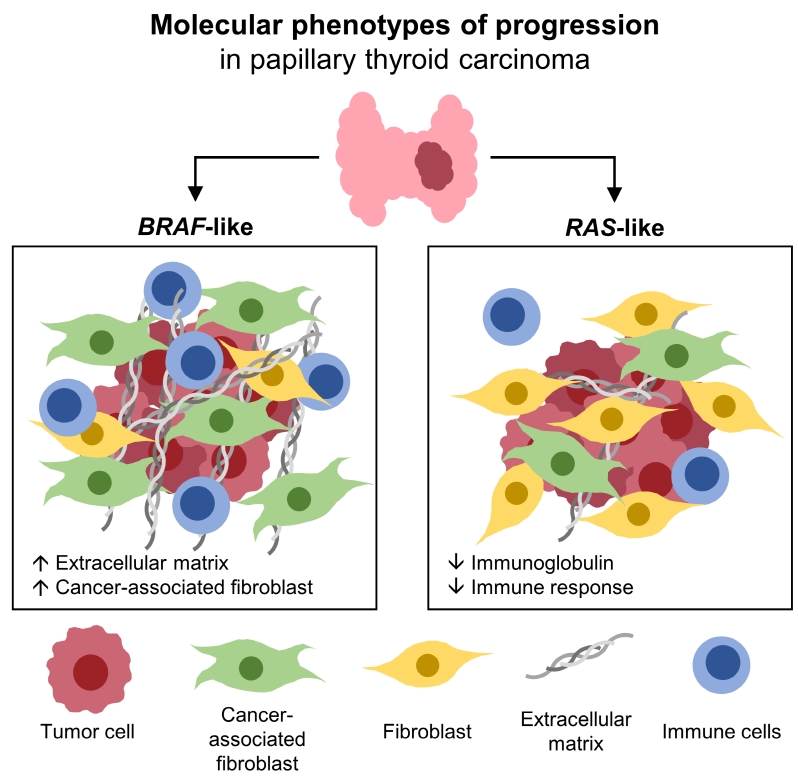
Even for the same clinicopathological factors, overlapping DEGs between the two molecular subtypes were uncommon, indicating that BL-PTCs and RL-PTCs have different progression mechanisms. Genes related to the extracellular matrix were commonly upregulated in BL-PTCs with aggressive clinicopathological factors, such as old age (≥55 years), presence of extrathyroidal extension, lymph node metastasis, advanced tumor-node-metastasis (TNM) stage, and high metastasis-age-completeness of resection- invasion-size (MACIS) scores (≥6). Furthermore, in the deconvolution analysis of tumor microenvironment, cancer-associated fibroblasts were significantly enriched. In contrast, in RL-PTCs, downregulation of immune response and immunoglobulin-related genes was significantly associated with aggressive characteristics, even after adjusting for thyroiditis status.
Conclusion
The molecular phenotypes of cancer progression differed between BL-PTC and RL-PTC. In particular, extracellular matrix and cancer-associated fibroblasts, which constitute the tumor microenvironment, would play an important role in the progression of BL-PTC that accounts for the majority of advanced PTCs.
-
Citations
Citations to this article as recorded by  - Papillary Thyroid Cancer Remodels the Genetic Information Processing Pathways
Dumitru Andrei Iacobas, Sanda Iacobas
Genes.2024; 15(5): 621. CrossRef
- Diabetes, Obesity and Metabolism
Big Data Articles (National Health Insurance Service Database)
- The Clinical Characteristics of Gestational Diabetes Mellitus in Korea: A National Health Information Database Study
-
Kyung-Soo Kim, Sangmo Hong, Kyungdo Han, Cheol-Young Park
-
Endocrinol Metab. 2021;36(3):628-636. Published online May 26, 2021
-
DOI: https://doi.org/10.3803/EnM.2020.948
-
-
5,921
View
-
173
Download
-
11
Web of Science
-
13
Crossref
-
 Abstract Abstract
 PDF PDF Supplementary Material Supplementary Material PubReader PubReader  ePub ePub
- Background
To investigate the clinical characteristics of gestational diabetes mellitus (GDM) in Korea, using a nationwide database.
Methods
We analyzed 417,139 women who gave birth between 2011 and 2015 using the Korean National Health Information Database. They underwent the Korean National Health Screening Program within one year before pregnancy and were not prescribed drugs for diabetes nor diagnosed with diabetes mellitus before 280 days antepartum. Patients with GDM were defined as those who visited the outpatient clinic more than twice with GDM codes.
Results
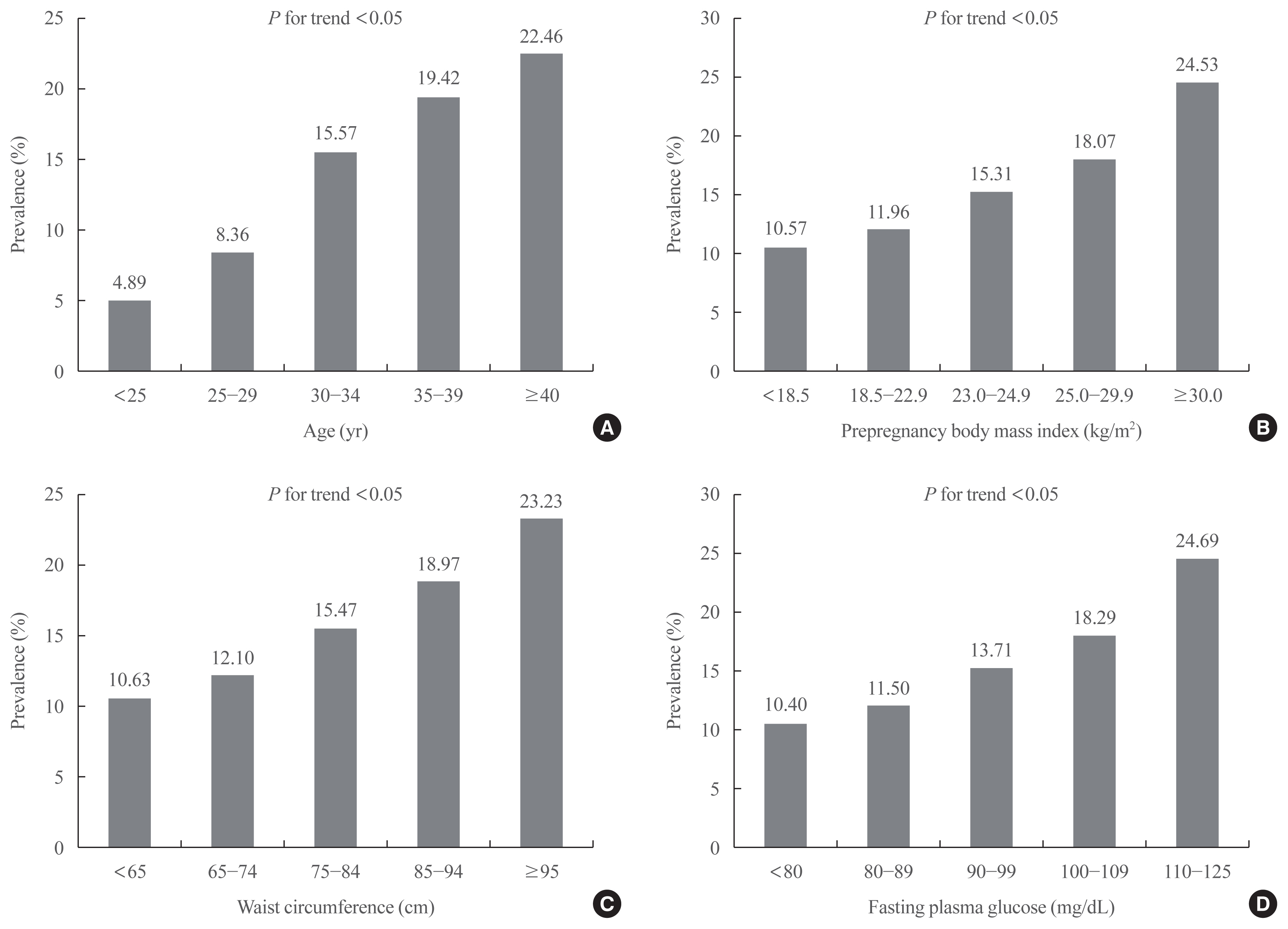
The prevalence of GDM was 12.70% and increased with increasing maternal age, prepregnancy body mass index (BMI), waist circumference (WC), and fasting plasma glucose (FPG) (P for trend <0.05). As compared with those aged <25 years, the odds ratio for women with GDM aged ≥40 years were 4.804 (95% confidence interval [CI], 4.436 to 5.203) after adjustment for covariates. Women with prepregnancy BMI ≥30 kg/m2 were at 1.898 times (95% CI, 1.736 to 2.075) greater risk for GDM than those with prepregnancy BMI <18.5 kg/m2. Women with WC of ≥95 cm were at 1.158 times (95% CI, 1.029 to 1.191) greater risk for GDM than women with WC of less than 65 cm. High FPG, high income, smoking, and drinking were associated with an elevated risk of GDM.
Conclusion
The prevalence of GDM in Korean women increased up to 12.70% during 2011 to 2015. These data suggest the importance of GDM screening and prevention in high-risk groups in Korea.
-
Citations
Citations to this article as recorded by  - Relationships between triglyceride-glucose index and incident gestational diabetes mellitus: a prospective cohort study of a Korean population using publicly available data
Zihe Mo, Changchun Cao, Yong Han, Haofei Hu, Yongcheng He, Xin Zuo
Frontiers in Public Health.2024;[Epub] CrossRef - Glucose tolerance test with a single abnormal value as a predictor of type 2 diabetes mellitus: a multicenter retrospective study
Seon Ui Lee, Subeen Hong, Sae Kyung Choi, Su Mi Kim, Jae Eun Shin, Ki Cheol Kil, Yeon Hee Kim, Jeong Ha Wie, Yun Sung Jo, Hyun Sun Ko
Scientific Reports.2024;[Epub] CrossRef - Exploring the influence of microbiota on gestational diabetes and its potential as a biomarker
Suresh Bokoliya, Stephanie McClellan, Yanjiao Zhou, Nini Fan
Frontiers in Bacteriology.2024;[Epub] CrossRef - Association Analysis of Dietary Inflammatory Index and Gestational
Diabetes Mellitus: Based on National Health and Nutrition Examination Survey
Database
Yamin Zeng, Yina Piao
Experimental and Clinical Endocrinology & Diabetes.2024;[Epub] CrossRef - Serum afamin levels in predicting gestational diabetes mellitus and preeclampsia: A systematic review and meta-analysis
Ying Yuan, Wenyin He, Xuejiao Fan, Junyu Liang, Zhen Cao, Lei Li
Frontiers in Endocrinology.2023;[Epub] CrossRef - Smoking during pregnancy and gestational diabetes mellitus: a systematic review and meta-analysis
Kleoniki I. Athanasiadou, Stavroula A. Paschou, Evgenia Papakonstantinou, Vasiliki Vasileiou, Fotini Kanouta, Paraskevi Kazakou, Katerina Stefanaki, Georgia N. Kassi, Theodora Psaltopoulou, Dimitrios G. Goulis, Eleni Anastasiou
Endocrine.2023; 82(2): 250. CrossRef - Association between the triglyceride to high-density lipoprotein cholesterol ratio and the risk of gestational diabetes mellitus: a second analysis based on data from a prospective cohort study
Yun You, Haofei Hu, Changchun Cao, Yong Han, Jie Tang, Weihua Zhao
Frontiers in Endocrinology.2023;[Epub] CrossRef - Effects of early standardized management on the growth trajectory of offspring with gestational diabetes mellitus at 0–5 years old: a preliminary longitudinal study
Bingbing Guo, Jingjing Pei, Yin Xu, Yajie Wang, Xinye Jiang
Scientific Reports.2023;[Epub] CrossRef - The Benefits Of Continuous Glucose Monitoring In Pregnancy
Jee Hee Yoo, Jae Hyeon Kim
Endocrinology and Metabolism.2023; 38(5): 472. CrossRef - Gestational Diabetes Mellitus: Diagnostic Approaches and Maternal-Offspring Complications
Joon Ho Moon, Hak Chul Jang
Diabetes & Metabolism Journal.2022; 46(1): 3. CrossRef - Current Trends of Big Data Research Using the Korean National Health Information Database
Mee Kyoung Kim, Kyungdo Han, Seung-Hwan Lee
Diabetes & Metabolism Journal.2022; 46(4): 552. CrossRef - Maternal Gestational Diabetes Influences DNA Methylation in the Serotonin System in the Human Placenta
Jae Yen Song, Kyung Eun Lee, Eun Jeong Byeon, Jieun Choi, Sa Jin Kim, Jae Eun Shin
Life.2022; 12(11): 1869. CrossRef - Fetal Abdominal Obesity Detected At 24 to 28 Weeks of Gestation Persists Until Delivery Despite Management of Gestational Diabetes Mellitus (Diabetes Metab J 2021;45:547-57)
Kyung-Soo Kim
Diabetes & Metabolism Journal.2021; 45(6): 966. CrossRef
- Clinical Study
- Vitamin D Deficiency at Mid-Pregnancy Is Associated with a Higher Risk of Postpartum Glucose Intolerance in Women with Gestational Diabetes Mellitus
-
Kyung-Soo Kim, Seok Won Park, Yong-Wook Cho, Soo-Kyung Kim
-
Endocrinol Metab. 2020;35(1):97-105. Published online March 19, 2020
-
DOI: https://doi.org/10.3803/EnM.2020.35.1.97
-
-
5,629
View
-
117
Download
-
9
Web of Science
-
9
Crossref
-
 Abstract Abstract
 PDF PDF PubReader PubReader  ePub ePub
- Background
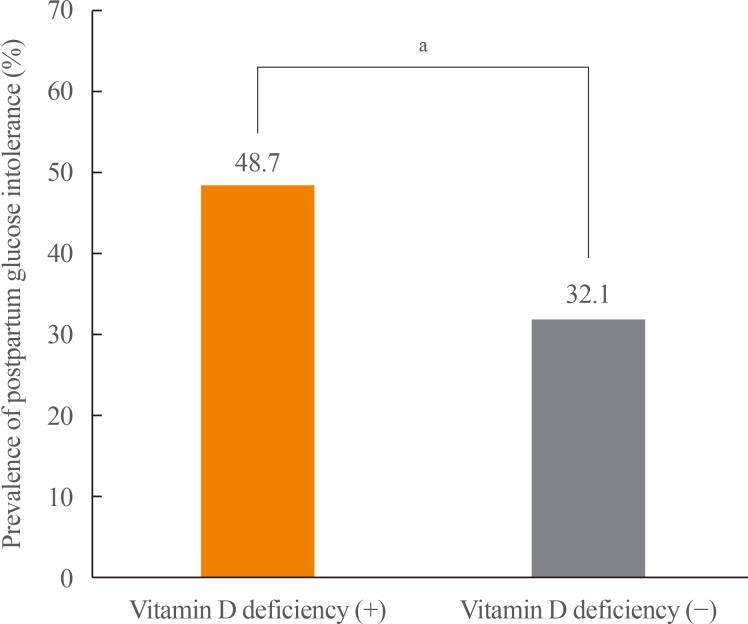
To evaluate the association between serum 25-hydroxyvitamin D (25(OH)D) at mid-pregnancy and postpartum glucose intolerance in women with gestational diabetes mellitus (GDM). MethodsWe enrolled 348 pregnant women diagnosed with GDM from August 2012 to October 2016. We measured serum 25(OH)D levels at mid-pregnancy and carried out a 75-g oral glucose tolerance test at 6 to 12 weeks after delivery. Vitamin D deficiency was defined as serum 25(OH)D <20 ng/mL. ResultsThe prevalence of vitamin D deficiency was 76.7% (n=267). Women with vitamin D deficiency had a higher prevalence of postpartum glucose intolerance than did those without vitamin D deficiency (48.7% vs. 32.1%, P=0.011). Serum 25(OH)D level was negatively correlated with hemoglobin A1c at antepartum and postpartum period (antepartum: r=−0.186, P=0.001; postpartum: r=−0.129, P=0.047). Homeostasis model assessment of β-cell function was positively correlated with serum 25(OH)D level only postpartum (r=0.138, P=0.035). The risk of postpartum glucose intolerance was 2.00 times (95% confidence interval, 1.13 to 3.55) higher in women with vitamin D deficiency than in those without vitamin D deficiency (P=0.018). ConclusionIn women with GDM, vitamin D deficiency at mid-pregnancy is associated with an elevated risk of postpartum glucose intolerance.
-
Citations
Citations to this article as recorded by  -
Evaluating the effect of vitamin D
3
fortification on physicochemical and sensory properties of yogurt
Saneela Saleem, Zahra Khan, Imtiaz Hussain, Faran Khan, Fahad Al-Asmari, Faima Atta Khan, Alyan Ali Zafar, Muhammad Abdul Rahim, Zongo Eliasse, Mohamed Fawzy Ramadan
Cogent Food & Agriculture.2024;[Epub] CrossRef - Vitamin D Supplementation for the Outcomes of Patients with Gestational Diabetes Mellitus and Neonates: A Meta-Analysis and Systematic Review
Chunfeng Wu, Yang Song, Xueying Wang, Pier P. Sainaghi
International Journal of Clinical Practice.2023; 2023: 1. CrossRef - Influence of hypovitaminosis D during pregnancy on glycemic and lipid profile, inflammatory indicators and anthropometry of pregnant and newborn
Sara de Figueiredo dos Santos, Paula Normando dos Reis Costa, Thaise Gasser Gouvêa, Nathalia Ferreira Antunes de Almeida, Felipe de Souza Cardoso
Clinical Nutrition ESPEN.2023; 54: 81. CrossRef - Risk factors associated with early postpartum glucose intolerance in women with a history of gestational diabetes mellitus: a systematic review and meta-analysis
Zhe Liu, Qianghuizi Zhang, Leyang Liu, Weiwei Liu
Endocrine.2023; 82(3): 498. CrossRef - Postprandial Free Fatty Acids at Mid-Pregnancy Increase the Risk of Large-for-Gestational-Age Newborns in Women with Gestational Diabetes Mellitus
So-Yeon Kim, Young Shin Song, Soo-Kyung Kim, Yong-Wook Cho, Kyung-Soo Kim
Diabetes & Metabolism Journal.2022; 46(1): 140. CrossRef - Effect of Evidence-Based Diet Nursing on Intestinal Flora and Maternal and Infant Prognosis in Patients with Gestational Diabetes
Ying Jiang, Chunbo Qiu, Yuanping Wang, Bin He, Peng-Yue Zhang
Evidence-Based Complementary and Alternative Medicine.2022; 2022: 1. CrossRef - Vitamin D in gestational diabetes: A broadened frontier
Yu Zhu, Ling Li, Ping Li
Clinica Chimica Acta.2022; 537: 51. CrossRef - The Clinical Characteristics of Gestational Diabetes Mellitus in Korea: A National Health Information Database Study
Kyung-Soo Kim, Sangmo Hong, Kyungdo Han, Cheol-Young Park
Endocrinology and Metabolism.2021; 36(3): 628. CrossRef - Fetal Abdominal Obesity Detected At 24 to 28 Weeks of Gestation Persists Until Delivery Despite Management of Gestational Diabetes Mellitus (Diabetes Metab J 2021;45:547-57)
Kyung-Soo Kim
Diabetes & Metabolism Journal.2021; 45(6): 966. CrossRef
- Clinical Study
- Obesity and Hyperglycemia in Korean Men with Klinefelter Syndrome: The Korean Endocrine Society Registry
-
Seung Jin Han, Kyung-Soo Kim, Wonjin Kim, Jung Hee Kim, Yong-ho Lee, Ji Sun Nam, Ji A Seo, Bu Kyung Kim, Jihyun Lee, Jin Ook Chung, Min-Hee Kim, Tae-Seo Sohn, Han Seok Choi, Seong Bin Hong, Yoon-Sok Chung
-
Endocrinol Metab. 2016;31(4):598-603. Published online December 20, 2016
-
DOI: https://doi.org/10.3803/EnM.2016.31.4.598
-
-
5,355
View
-
35
Download
-
20
Web of Science
-
18
Crossref
-
 Abstract Abstract
 PDF PDF PubReader PubReader
- Background
The aim of this study was to investigate the prevalence of obesity in Korean men with Klinefelter syndrome (KS) and the associated risk factors for obesity and hyperglycemia. MethodsData were collected retrospectively from medical records from 11 university hospitals in Korea between 1994 and 2014. Subjects aged ≥18 years with newly diagnosed KS were enrolled. The following parameters were recorded at baseline before treatment: chief complaint, height, weight, fasting glucose level, lipid panel, blood pressure, testosterone, luteinizing hormone, follicle-stimulating hormone, karyotyping patterns, and history of hypertension, diabetes, and dyslipidemia. ResultsData were analyzed from 376 of 544 initially enrolled patients. The rate of the 47 XXY chromosomal pattern was 94.1%. The prevalence of obesity (body mass index ≥25 kg/m2) in Korean men with KS was 42.6%. The testosterone level was an independent risk factor for obesity and hyperglycemia. ConclusionObesity is common in Korean men with KS. Hypogonadism in patients with KS was associated with obesity and hyperglycemia.
-
Citations
Citations to this article as recorded by  - A dual-center study of predictive factors for sperm retrieval through microdissection testicular sperm extraction and intracytoplasmic sperm injection outcomes in men with non-mosaic Klinefelter syndrome
Jong Hyeun Baeck, Tae Jin Kim, Tae Heon Kim, Seung-Ryeol Lee, Dong Soo Park, Hwang Kwon, Ji Eun Shin, Dong Hyeon Lee, Young Dong Yu
Investigative and Clinical Urology.2023; 64(1): 41. CrossRef - Cardiorespiratory fitness in adolescents and young adults with Klinefelter syndrome – a pilot study
Julia Spiekermann, Kathrin Sinningen, Beatrice Hanusch, Michaela Kleber, Michael M. Schündeln, Cordula Kiewert, Heide Siggelkow, Jakob Höppner, Corinna Grasemann
Frontiers in Endocrinology.2023;[Epub] CrossRef - Metabolic Profile in a Cohort of Young Sicilian Patients with Klinefelter’s Syndrome: The Role of Irisin
Stefano Radellini, Valentina Guarnotta, Vincenzo Sciabica, Giuseppe Pizzolanti, Carla Giordano, Vito Angelo Giagulli
International Journal of Endocrinology.2022; 2022: 1. CrossRef - Metabolic and Nutritional Aspects in Paediatric Patients with Klinefelter Syndrome: A Narrative Review
Chiara Mameli, Giulia Fiore, Arianna Sangiorgio, Marta Agostinelli, Giulia Zichichi, Gianvincenzo Zuccotti, Elvira Verduci
Nutrients.2022; 14(10): 2107. CrossRef - Klinefelter syndrome in an adolescent with severe obesity, insulin resistance, and hyperlipidemia, successfully treated with testosterone replacement therapy
Shota Fukuhara, Jun Mori, Hisakazu Nakajima
Clinical Pediatric Endocrinology.2021; 30(3): 127. CrossRef - Glucose metabolic disorder in Klinefelter syndrome: a retrospective analysis in a single Chinese hospital and literature review
Shixuan Liu, Tao Yuan, Shuoning Song, Shi Chen, Linjie Wang, Yong Fu, Yingyue Dong, Yan Tang, Weigang Zhao
BMC Endocrine Disorders.2021;[Epub] CrossRef - What Every Internist-Endocrinologist Should Know about Rare Genetic Syndromes in Order to Prevent Needless Diagnostics, Missed Diagnoses and Medical Complications: Five Years of ‘Internal Medicine for Rare Genetic Syndromes’
Anna G. W. Rosenberg, Minke R. A. Pater, Karlijn Pellikaan, Kirsten Davidse, Anja A. Kattentidt-Mouravieva, Rogier Kersseboom, Anja G. Bos-Roubos, Agnies van Eeghen, José M. C. Veen, Jiske J. van der Meulen, Nina van Aalst-van Wieringen, Franciska M. E. H
Journal of Clinical Medicine.2021; 10(22): 5457. CrossRef - Klinefelter Syndrome and Diabetes
Mark J. O’Connor, Emma A. Snyder, Frances J. Hayes
Current Diabetes Reports.2019;[Epub] CrossRef - Endocrine aspects of Klinefelter syndrome
Adriana Herrera Lizarazo, Michelle McLoughlin, Maria G. Vogiatzi
Current Opinion in Endocrinology, Diabetes & Obesity.2019; 26(1): 60. CrossRef - Sex differences in metabolism and cardiometabolic disorders
Karthickeyan Chella Krishnan, Margarete Mehrabian, Aldons J. Lusis
Current Opinion in Lipidology.2018; 29(5): 404. CrossRef - Klinefelter Syndrome: Integrating Genetics, Neuropsychology, and Endocrinology
Claus H Gravholt, Simon Chang, Mikkel Wallentin, Jens Fedder, Philip Moore, Anne Skakkebæk
Endocrine Reviews.2018; 39(4): 389. CrossRef - Sex differences in obesity, lipid metabolism, and inflammation—A role for the sex chromosomes?
Temeka Zore, Maria Palafox, Karen Reue
Molecular Metabolism.2018; 15: 35. CrossRef - Klinefelter syndrome, insulin resistance, metabolic syndrome, and diabetes: review of literature and clinical perspectives
Andrea Salzano, Roberta D’Assante, Liam M. Heaney, Federica Monaco, Giuseppe Rengo, Pietro Valente, Daniela Pasquali, Eduardo Bossone, Daniele Gianfrilli, Andrea Lenzi, Antonio Cittadini, Alberto M. Marra, Raffaele Napoli
Endocrine.2018; 61(2): 194. CrossRef - Síndrome de Klinefelter y riesgo cardiovascular
A. Yamaguchi, P. Knoblovits
Hipertensión y Riesgo Vascular.2018; 35(4): 195. CrossRef - Articles inEndocrinology and Metabolismin 2016
Won-Young Lee
Endocrinology and Metabolism.2017; 32(1): 62. CrossRef - Sex differences in obesity: X chromosome dosage as a risk factor for increased food intake, adiposity and co-morbidities
Karen Reue
Physiology & Behavior.2017; 176: 174. CrossRef - Klinefelter Syndrome with Morbid Obesity Before Bariatric Surgery: A Case Report
Parisa Janmohammadi, Gholamreza Mohammadi-Farsani, Hana Arghavani, Mahmoud Arshad, Tayebeh Mokhber
Journal of Minimally Invasive Surgical Sciences.2017;[Epub] CrossRef - Klinefelter Syndrome and Metabolic Disorder
Ji Cheol Bae
Endocrinology and Metabolism.2016; 31(4): 535. CrossRef
- Obesity and Metabolism
- Low Serum Testosterone Concentrations in Hospitalized Men with Poorly Controlled Type 2 Diabetes
-
Kyung-Soo Kim, San-Ha Kang, Moon-Jong Kim, Soo-Kyung Kim, Yoo-Lee Kim, Won-Keun Park, Seok Won Park, Yong-Wook Cho
-
Endocrinol Metab. 2014;29(4):574-578. Published online December 29, 2014
-
DOI: https://doi.org/10.3803/EnM.2014.29.4.574
-
-
3,396
View
-
31
Download
-
6
Web of Science
-
6
Crossref
-
 Abstract Abstract
 PDF PDF PubReader PubReader
Our aim was to examine whether serum testosterone concentrations are in fact low in hospitalized men with poorly controlled type 2 diabetes compared with healthy men. In this study, 79 men aged 40 years or older (41 healthy men and 38 men with type 2 diabetes) were included. Total testosterone and sex hormone-binding globulin levels were measured. The average duration of diagnosed diabetes was 10.8 years and the mean glycated hemoglobin value was 10.8%. Total testosterone concentrations were lower in men with type 2 diabetes than in healthy men, after adjusting for age and body mass index (3.83±0.32 ng/mL vs. 5.63±0.31 ng/mL, P<0.001). In conclusion, this study shows that serum testosterone concentrations are lower in hospitalized men with poorly controlled type 2 diabetes than in healthy men. Therefore, men with poorly controlled type 2 diabetes should undergo further assessment for hypogonadism. -
Citations
Citations to this article as recorded by  - Nanoparticles of Costus speciosus Ameliorate Diabetes-Induced Structural Changes in Rat Prostate through Mediating the Pro-Inflammatory Cytokines IL 6, IL1β and TNF-α
Duaa Bakhshwin, Khadija Abdul Jalil Faddladdeen, Soad Shaker Ali, Samar Mohammed Alsaggaf, Nasra Naeim Ayuob
Molecules.2022; 27(3): 1027. CrossRef - Association between testosterone with type 2 diabetes in adult males, a meta-analysis and trial sequential analysis
Jianzhong Zhang, Xiao Li, Zhonglin Cai, Hongjun Li, Bin Yang
The Aging Male.2020; 23(5): 607. CrossRef - Momordica charantia Extract Protects against Diabetes-Related Spermatogenic Dysfunction in Male Rats: Molecular and Biochemical Study
Gamal A. Soliman, Rehab F. Abdel-Rahman, Hanan A. Ogaly, Hassan N. Althurwi, Reham M. Abd-Elsalam, Faisal F. Albaqami, Maged S. Abdel-Kader
Molecules.2020; 25(22): 5255. CrossRef - Olive leaves extract attenuates type II diabetes mellitus-induced testicular damage in rats: Molecular and biochemical study
Gamal A. Soliman, Abdulaziz S. Saeedan, Rehab F. Abdel-Rahman, Hanan A. Ogaly, Reham M. Abd-Elsalam, Maged S. Abdel-Kader
Saudi Pharmaceutical Journal.2019; 27(3): 326. CrossRef - Effects of testosterone supplement treatment in hypogonadal adult males with T2DM: a meta-analysis and systematic review
Jianzhong Zhang, Bin Yang, Wenhui Xiao, Xiao Li, Hongjun Li
World Journal of Urology.2018; 36(8): 1315. CrossRef - RAS and sex differences in diabetic nephropathy
Sergi Clotet, Marta Riera, Julio Pascual, María José Soler
American Journal of Physiology-Renal Physiology.2016; 310(10): F945. CrossRef
|